ISO13485
The quality management
system ISO13485:2016 , issued by NQA ( UKAS), specially for the micro needling
system and electric micro needling system.
Certificate includes
factory annual inspection, also with the inspection report( Chinese
version).Based on the certificate, manufacturer is available to issue the
inspection report of the finished product, as well as the product sterilization
report according to the customer demands.

CE1282
& ROHS
Issued
by ECM ,at medical device directive 93/42/EEC, technical stands: EN ISO10993-1:2009 EN ISO10993-10:2013 EN ISO10993-12:2012.
This
certificate is for passive products, that means the products without electric
power.
ROSH
at directive IEC 62321-5:2013 IEC
62321-4:2013 IEC 62321:2008 IEC 62321-6:2015


INMETRO
The
necessary certificate that export to Brazil market. Specially for the active
products, which means the product with
electric power.
Tested and reported by
SGS, technical stands: IEC60601-1k
IEC60601-1-11c
IEC60601-1-2:2007 IEC60601-1-6h
The
adaptor approved by UL and FCC ( for US/EU/UK plug), EN14136 ( special for
Brazil market plug)
Based
on this certificate, and SGS demands, special inspections are necessary, which
includes : Type of high-voltage insulation test Records and Type of Leakage
Current and Test Condition Records

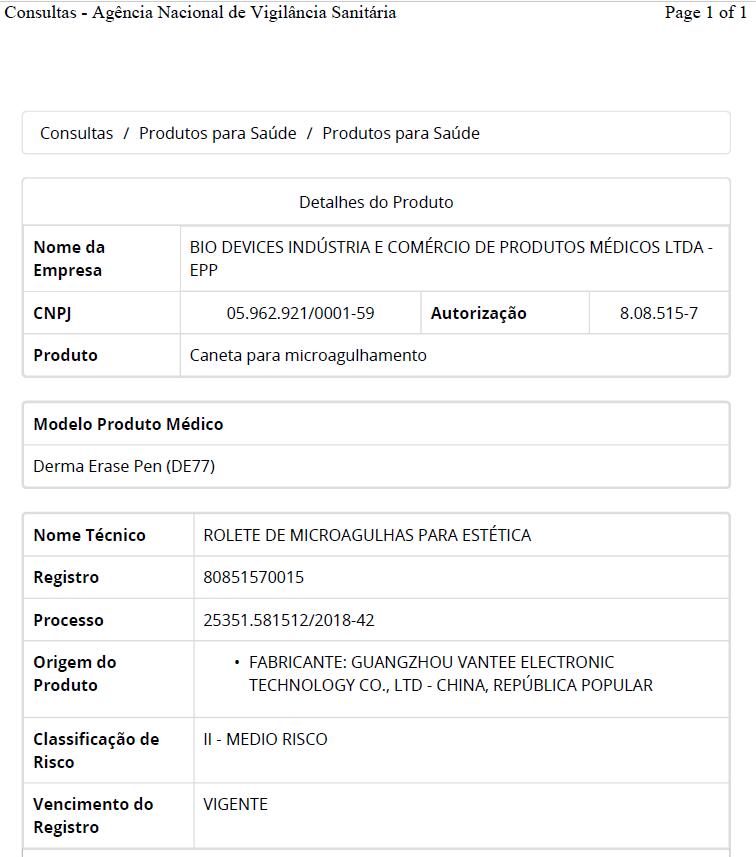
ANVISA
The necessary
certificate that export to Brazil market.
EU-REP
European Authorized
Representative
Company: SUNGO Europe B.V.
Add: Olympisch Stadion 24, 1076DE,
Amsterdam, Netherlands
Email: EC.REP@sungogroup.com
Phone: +31 08 5029 0204

CIBG
Product registration in
Europe.


Class ⅡMedical Device Business Certificate



Certificate
of Patent

